The Power of Foliar Feeding.
Why Foliar Feed?
Foliar feeding is a powerful way to provide crops with essential micronutrients throughout the growing season. When done properly, foliar fertilization can be more target oriented compared to soil-applied methods, since nutrients can be delivered directly to plant tissue during critical stages of plant growth. Although it doesn’t replace soil nutrition, foliar nutrition can complement these existing programs to increase yield potential and/or quality across a number of crops. Foliar fertilizers are commonly used to:
- Correct a deficiency – to improve plant health and development when nutrient uptake from the roots is suppressed or delayed.
- Decrease stress – to alleviate stress before or after exposure to unfavourable conditions.
- Optimize production – supplement nutrient supplies curing critical peak demand periods
Evidence for nutrient uptake.
One of the most common misconceptions about foliar fertilization is doesn’t make a difference since leaves aren’t designed to take up nutrients. However, much evidence exists for foliar uptake of nutrients, among the most impressive being the response of perennial crops when growth-limiting nutrients have been applied to half of the tree resulting in a lush green canopy on the treated-side, whereas the other side of the trees present severe deficiency symptoms (Figure 1).
The image below shows a nickel deficiency in a pecan tree growing on sandy, low nickel soil in Georgia. The left side of the tree was spray with a solution of NiSO4 just before leafing out in spring, which corrected the deficiency. The right side was not sprayed with nickel. The close-up view reveals characteristic Ni deficiency symptoms, a malformation called “mouse-ear” due to the shape of the stunted, malformed leaves.
Other evidence includes increased nutrient concentration in sprayed tissue, yield, and/or crop quality. Mitigation of Ca-related disorders, particularly bitter pit in apple fruit, has been repeatedly demonstrated with foliar application of calcium salts, and with lower rates of calcium applied as chelated formulations.

Figure 1. Nickel-deficient Pecan Tree showing normal foliage where a foliar spray was applied on the left side1
Foliar-applied fertilizers can enter the plant through several different pathways, via the cuticle along cuticular cracks/imperfections or through structures like stomata, trichomes or lenticels. Penetration into the stomata depends on applying the foliar when they are open and requires a surfactant to decrease surface tension. Stomata are typically more numerous on the underside of leaves and may be difficult to target with sprayers.
Research has demonstrated another direct pathway, called aqueous or polar pores, for movement of hydrophilic solutes through the waxy cuticle that covers the leaves. This pathway may be a result of adsorption of water onto the polar regions of molecules within the cuticular layer. In general, these polar pores are abundant at cuticular ledges of stomata, above anticlinal cell walls and at the base of trichomes (Figure 2). Higher humidity causes the cuticle to swell and can increase the frequency of polar pores by two to three times. Indirect approaches to measure the diameter of these pores has provided a range of sizes from 1 to 5 nm, depending on the species.
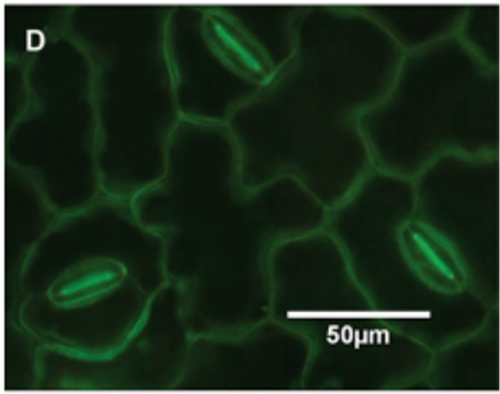
Figure 2. Look down on a leaf surface. Green = Rich in Polar Pores.
The form of the nutrient.
To successfully pass through polar pores, foliar fertilizers must meet three main criteria. They must be small (<5 nm), neutral and they must be true solutions. NutriAg’s PAC carbohydrate chelates meet all three of these criteria. Plant cuticles are negatively charged, therefore uncharged or neutral compounds can penetrate the plant and can be translocated in the apoplast easier than positively charged cations. Suspension products contain nutrients that are not fully dissolved which can cause a range of issues such as clogging sprayers. While a small proportion of the applied nutrient may be in solution and pass through the cuticle, the vast majority become deposited on the leaf surface as the spray dries and remains unavailable to the crop (Figure 3). Foliar fertilizers based on salts are often used and can be effective, however salts disassociate into positive and negative ions, hindering the ability of the cation to pass through the cuticle; salt-based formulations also have a tendency to burn the crop.
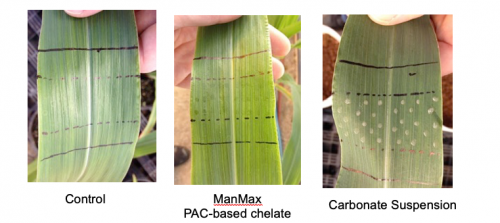
Figure 3. Equal amounts of manganese were applied to corn leaves, the suspension product is sitting on the leaf surface.
Using chelates to form true solutions is a more effective method for foliar fertilization. Chelates are compounds that bind to metals and form a strong, ring-like structure. A negatively charged ligand with multiple binding sides interacts with a positively charged nutrient ion, neutralizing the charge and forming a chelate (Figure 3). Chelates also prevent interaction with other ions in solution, they significantly improve solubility and compatibility in the tank, and they allow for improved uptake by the leaf. NutriAg’s PAC technology uses plant-derived carbohydrates chelate essential crop nutrients to enhance uptake and optimize usage by the plant.
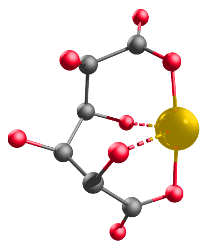
Figure 4. Chelate structure.
There are two types of chelates, synthetic and naturally occurring. Common synthetic chelates include EDTA, EDDHA and HEDTA. Synthetic chelates are often not recognized by the plant and are therefore poorly metabolized. EDTA is also very strong chelate which means it will not release the metal ion easily resulting in less efficient foliar nutrient uptake and utilization. Using carbohydrates as chelates is a more effective way to delivered foliar nutrients to the crop. They are naturally occurring and therefore easily recognized and metabolized by the plant. NutriAg’s exclusive PAC™ technology uses natural, plant carbohydrates to deliver fully chelated crop nutrition in a true solution.
Sources
- Wood, B.W., Reilly, C.C., and Nyczepir, A.P. 2004. Mouse-ear of Pecan: A Nickel Deficiency. Hort Sci. 39(6): 1238-1242.





